Sierra117 wrote: ↑23 Oct 2019, 21:02
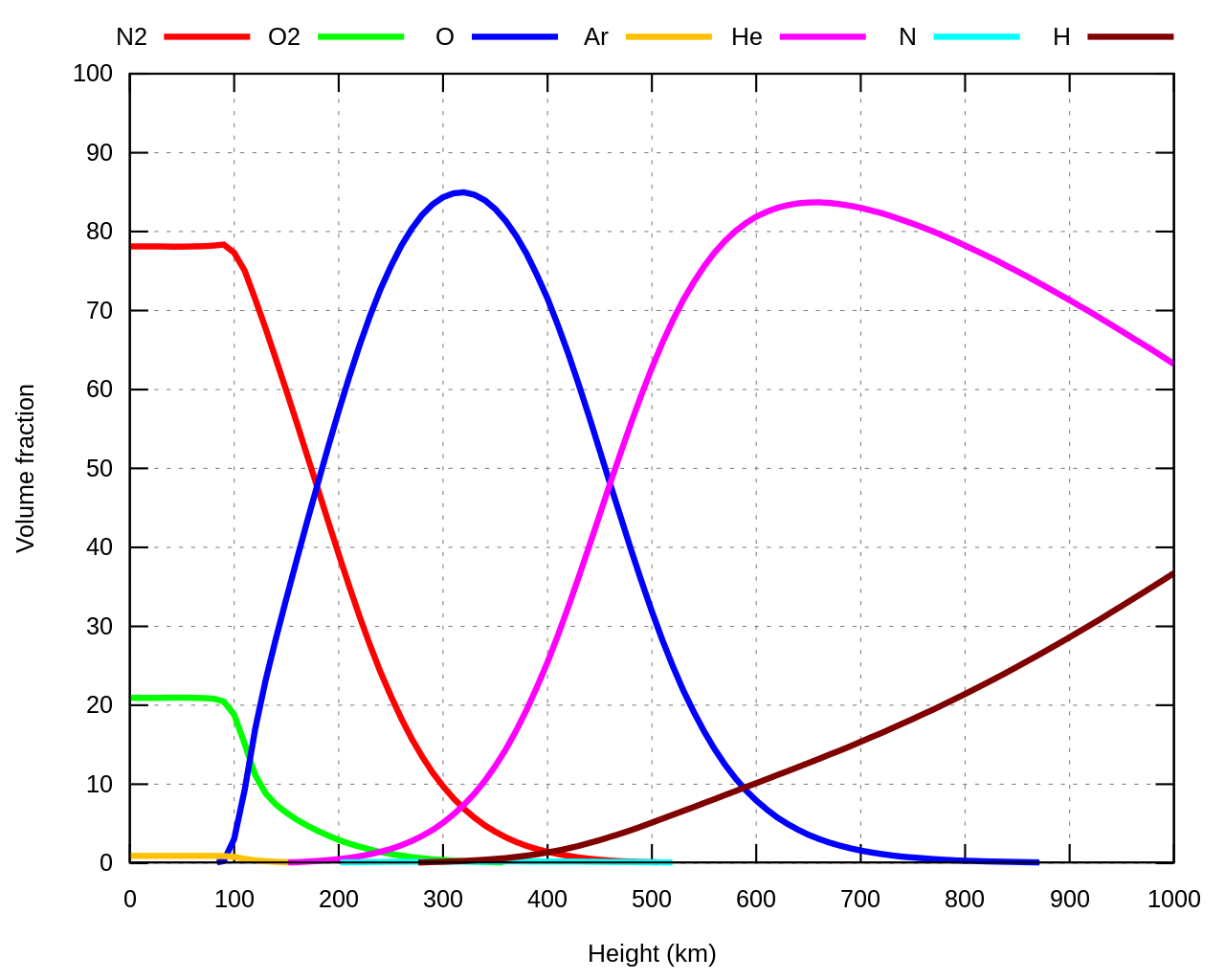
OT but could you explain the N molecule there in the chart? I thought it's unstable and we only have N2 but the chart even dedicated a line and colour to it and it can be seen a tiny bit on the chart at the bottom somewhere starting around 300km but remains flat.
The grapefruit smell can only mean there's some biofuel involvement. Maybe a particular compound that's isolated but also retains the smell. Like how the extract from fruit rind is used for its cleaning ability but the citrus smell carries over too.
Chemical elements which are in a gas state of matter exists as molecules rather than atoms, at ambient pressure and temperature, for being a more stable state of coexistence. Molecules, in general, are made by two atoms of the same chemical element, thus we could see terminology like O2, N2, H2 etc. and occasionally by 3 atoms (such as ozone) ...
At higher altitudes, there are lower temps and lower pressures and the chemical molecular bonds between the atoms are broken which led them to enter into an ionic state of matter, called plasma ... Thus, the terminology used is O2- "at the power of minus 2" or H+ which is a proton and so forth ... So, in that chart O, N, He, H are ions in a certain electric charging stage, plus (cations) or minus (anions).